You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
To The Moon
- Thread starter Declan
- Start date
More options
Who Replied?- Joined
- Sep 11, 2021
- Messages
- 9,803
- Reaction score
- 6,942

- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Exactly the point. They didn't. There will be no pics or footage of the moon lander on the actual moon. It's in a Hollywood warehouse somewhere. And apparently the Moon is full of rare earth minerals. You'd think there would be a commercial interest there.Why would they waste money on that when they already went there?
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Nobody taking pics of the moon while in orbit?
- Joined
- Mar 10, 2023
- Messages
- 1,086
- Reaction score
- 947
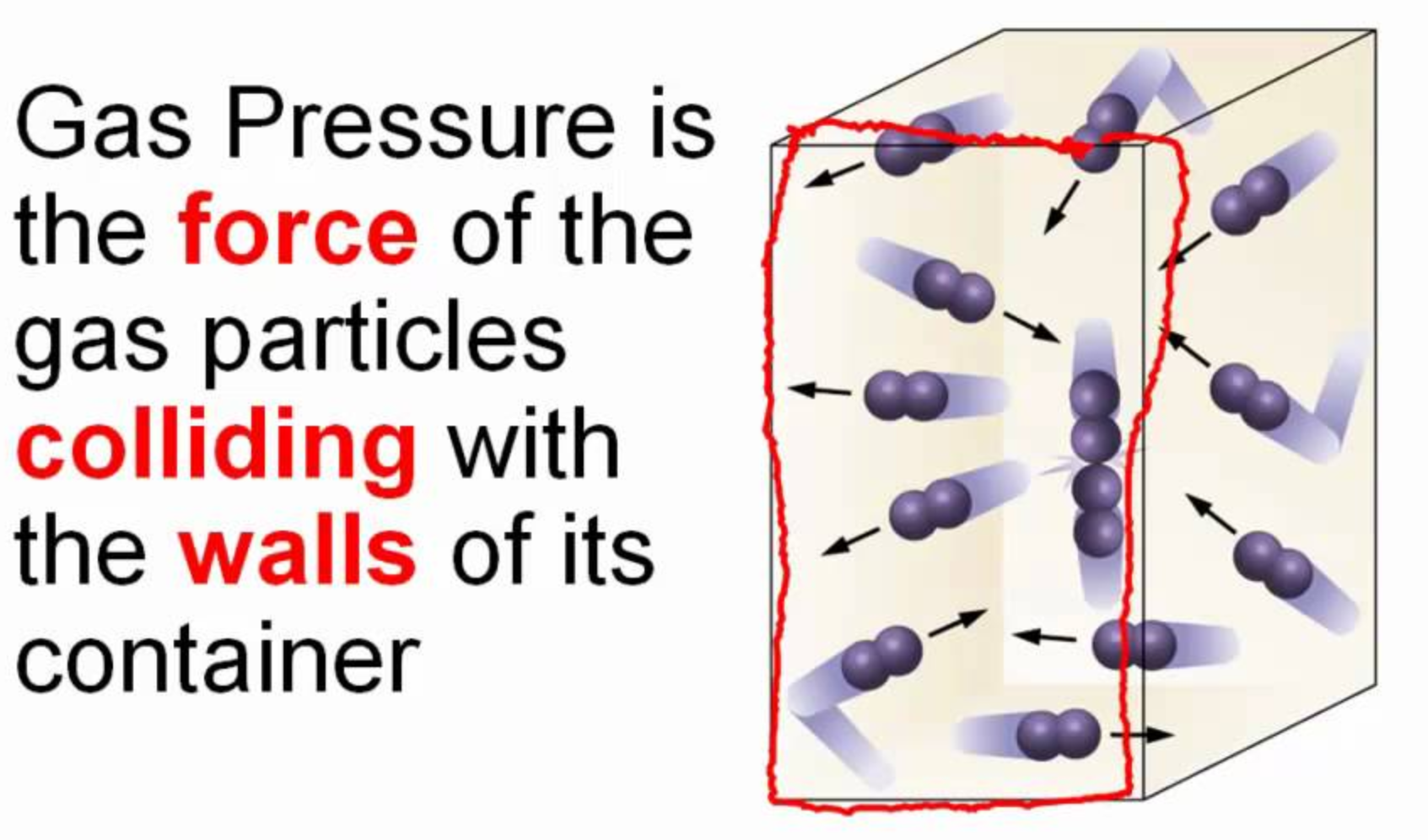
Gas pressure cannot exist next to a vacuum without a container. This has never been demonstrated. No container for the gas = nothing for the gas to press against = no pressure. We have air pressure on earth so that air MUST be contained.Sources for your point of view?(that's ok it's your personal view, fair enough) Actually it's your asserted POV for the Heliocentrician's that certainly require established sources of reference for us today.
Your claims here are beyond extraordinary surely, what proof this time?
There'll soon be much more up to date evidence available, things are going to get very busy
Earth's atmosphere (i.e. gas pressure) cannot exist next to the vacuum of space if there is no containment as per the globe model. Therefore the space vacuum is IMPOSSIBLE, no matter what any Government or space agency says.
From Florida State University Department of Chemistry & Biochemistry: https://www.chem.fsu.edu/chemlab/chm1045/gases.html
View: https://www.youtube.com/watch?v=NzKAJWTmlwg
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Gas pressure cannot exist next to a vacuum without a container. This has never been demonstrated. No container for the gas = nothing for the gas to press against = no pressure. We have air pressure on earth so that air MUST be contained.
Earth's atmosphere (i.e. gas pressure) cannot exist next to the vacuum of space if there is no containment as per the globe model. Therefore the space vacuum is IMPOSSIBLE, no matter what any Government or space agency says.
From Florida State University Department of Chemistry & Biochemistry: https://www.chem.fsu.edu/chemlab/chm1045/gases.html

View: https://www.youtube.com/watch?v=NzKAJWTmlwg
But but but...muh weak of force gravity, which can't even hold down a helium balloon, creates the perfect containment required for the official narrative to be real
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
The Russian lunar module has been in orbit since yesterday. Where are the photos of the US debris, Flag and moon buggy?
- Joined
- Mar 10, 2023
- Messages
- 1,086
- Reaction score
- 947
Flat earther confronts astrophysicists about gas pressure and vacuum - one guy "needs to go" when he realises he's fucked, the other remains silent:
View: https://www.youtube.com/watch?v=cHBSd18nIuw
View: https://www.youtube.com/watch?v=cHBSd18nIuw
- Joined
- Mar 10, 2023
- Messages
- 1,086
- Reaction score
- 947
@buddy love More sources on gas pressure for you:
To Invoke Gas Pressure WITHOUT a Container, you're merely VIOLATING ...
1. Boyle's Law – Relationship between pressure and volume in a gas at constant temperature.
2. Charles's Law – Relationship between volume and temperature of a gas at constant pressure.
3. Gay-Lusac's Law -- Relationship between pressure and temperature of a gas at constant volume.
4. Avagadro's Law - For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
5. Parts of Dalton’s Law: To summarize, the total pressure exerted by a mixture of gases is the sum of the partial pressures of component gases. This law was first discovered by John Dalton, the father of the atomic theory of matter. It is now known as Dalton’s law of partial pressures. Combined gas law (Ideal Gas Law) – Combination of Charles', Boyle's, and Gay-Lussac's gas laws. (PV=nRT)
6. Brownian Motion: "Real gas molecules move in all directions, not just to neighbors on a chessboard." http://web.mit.edu/8.334/www/grades/projects/projects17/OscarMickelin/brownian.html
7. 2nd Law of Thermodynamics: "The second law of thermodynamics can also be stated that "all spontaneous processes produce an increase in the entropy of the universe". https://chem.libretexts.org/Bookshe...f_Thermodynamics/Second_Law_of_Thermodynamics
"The "PRESSURE OF A GAS" is the force that the gas exerts on the 'WALLS OF ITS CONTAINER'". http://chemistry.elmhurst.edu/vchembook/180pressure.html
"The molecules are in constant, random motion and frequently collide with each other and with the WALLS OF A CONTAINER. Because the molecules are in motion, a GAS will expand to fill the CONTAINER. Since density is defined to be the mass divided by the volume, density DEPENDS DIRECTLY on the size of the CONTAINER in which a fixed mass of GAS is confined."
https://www.grc.nasa.gov/www/k-12/airplane/fluden.html
"Kinetic Molecular Theory Explanation of Boyle's [GAS] Law... "
Observations about pressure may be explained using the following ideas. The rapid motion and collisions of molecules with the WALLS OF THE CONTAINER "CAUSES" PRESSURE (force on a unit area). Pressure is proportional to the number of molecular collisions and the force of the collisions in a particular area. The more collisions of GAS MOLECULES with THE WALLS, the higher the PRESSURE."
http://chemistry.elmhurst.edu/vchembook/180pressure.html
"Pressure is 'CAUSED' by Gas Molecules hitting the WALLS OF THE CONTAINER. With a smaller VOLUME, the Gas Molecules will hit THE WALLS more frequently, and so the PRESSURE INCREASES. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Gases/Gas_Laws/Boyle's_Law
To Invoke Gas Pressure WITHOUT a Container, you're merely VIOLATING ...
1. Boyle's Law – Relationship between pressure and volume in a gas at constant temperature.
2. Charles's Law – Relationship between volume and temperature of a gas at constant pressure.
3. Gay-Lusac's Law -- Relationship between pressure and temperature of a gas at constant volume.
4. Avagadro's Law - For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
5. Parts of Dalton’s Law: To summarize, the total pressure exerted by a mixture of gases is the sum of the partial pressures of component gases. This law was first discovered by John Dalton, the father of the atomic theory of matter. It is now known as Dalton’s law of partial pressures. Combined gas law (Ideal Gas Law) – Combination of Charles', Boyle's, and Gay-Lussac's gas laws. (PV=nRT)
6. Brownian Motion: "Real gas molecules move in all directions, not just to neighbors on a chessboard." http://web.mit.edu/8.334/www/grades/projects/projects17/OscarMickelin/brownian.html
7. 2nd Law of Thermodynamics: "The second law of thermodynamics can also be stated that "all spontaneous processes produce an increase in the entropy of the universe". https://chem.libretexts.org/Bookshe...f_Thermodynamics/Second_Law_of_Thermodynamics
"The "PRESSURE OF A GAS" is the force that the gas exerts on the 'WALLS OF ITS CONTAINER'". http://chemistry.elmhurst.edu/vchembook/180pressure.html
"The molecules are in constant, random motion and frequently collide with each other and with the WALLS OF A CONTAINER. Because the molecules are in motion, a GAS will expand to fill the CONTAINER. Since density is defined to be the mass divided by the volume, density DEPENDS DIRECTLY on the size of the CONTAINER in which a fixed mass of GAS is confined."
https://www.grc.nasa.gov/www/k-12/airplane/fluden.html
"Kinetic Molecular Theory Explanation of Boyle's [GAS] Law... "
Observations about pressure may be explained using the following ideas. The rapid motion and collisions of molecules with the WALLS OF THE CONTAINER "CAUSES" PRESSURE (force on a unit area). Pressure is proportional to the number of molecular collisions and the force of the collisions in a particular area. The more collisions of GAS MOLECULES with THE WALLS, the higher the PRESSURE."
http://chemistry.elmhurst.edu/vchembook/180pressure.html
"Pressure is 'CAUSED' by Gas Molecules hitting the WALLS OF THE CONTAINER. With a smaller VOLUME, the Gas Molecules will hit THE WALLS more frequently, and so the PRESSURE INCREASES. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Gases/Gas_Laws/Boyle's_Law
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Any photos of the moon buggy and US Flag yet?
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Why are space cameras so shit? These, apparently, are Apollo landing site images from the Indian lunar orbiter. Seriously?? Someone give these lads an I-Phone 

- Joined
- Jun 14, 2023
- Messages
- 2,746
- Reaction score
- 2,638
Looks like a fly on cappuccino foam.Why are space cameras so shit? These, apparently, are Apollo landing site images from the Indian lunar orbiter. Seriously?? Someone give these lads an I-Phone
View attachment 2133
Probably is an iPhone
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Lol. An article about modern lunar photography has one well ropey, flat, stretched looking piece of obvious cgi. Why are these shots ALWAYS blurry ?
Chandrayaan-3: India's lunar lander Vikram sends close-up photos of Moon
The photos are taken by Vikram, Chandrayaan-3's lander, which has begun the last phase of its mission.www.bbc.com
Someone send these lads a modern Nikon please! Where's the US Flag and Rover??
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
I was looking at the fake lunar orbiter photos from the 1960s.
They are slightly BETTER than what we can manage today. Space is clearly fake.
They have created a visual style around faking these things, as a way to add a feeling of authenticity. This is generated using black and white images, blurred out, with a short angle lens and tight compositions.
They are slightly BETTER than what we can manage today. Space is clearly fake.
They have created a visual style around faking these things, as a way to add a feeling of authenticity. This is generated using black and white images, blurred out, with a short angle lens and tight compositions.
- Joined
- Jun 14, 2023
- Messages
- 2,746
- Reaction score
- 2,638
Do you remember a show that was shown on Channel 4 called ‘Space Cadets’. It was a reality to show where they convinced a bunch of morons that they win outer space, when in fact they were in a simulation machine in IpswichI was looking at the fake lunar orbiter photos from the 1960s.
They are slightly BETTER than what we can manage today. Space is clearly fake.
They have created a visual style around faking these things, as a way to add a feeling of authenticity. This is generated using black and white images, blurred out, with a short angle lens and tight compositions.
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
This was predicted by my good self of course. The Russian 'orbiter' has 'crashed on the moon' now, officially. More debris we will never see pics of.Russians in trouble!

Russia reports 'abnormal situation' at Luna-25 spacecraft
Russia is racing against India to make an ambitious landing on the moon's south pole, with its rival having launched its own lunar lander Chandrayaan-3 last month. Space agencies like NASA have detected frozen water in the area's craters before, but no country has ever ventured there.news.sky.com
Once again, 1960s tech beats 2023 tech.
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
We all space cadets, those of us that believe in space, and moon landings and complex, multidirectional earth orbits at thousands of miles per hour.Do you remember a show that was shown on Channel 4 called ‘Space Cadets’. It was a reality to show where they convinced a bunch of morons that they win outer space, when in fact they were in a simulation machine in Ipswich
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
My July 15 prophecy has come true:
 www.sarsfieldsvirtualpub.com
www.sarsfieldsvirtualpub.com
To The Moon
View: https://youtu.be/jSYiXWEjgcc Blast off is just after noon in Ireland on Monday. This bears watching as in my opinion, this is an attempt to send a human to the moon. They are to have many tests. Including getting radiation readings to see if man can go into space at all.
- Joined
- Sep 11, 2021
- Messages
- 9,803
- Reaction score
- 6,942
Well one is certainly kaput and the latest is that the Indian attempt is Tuesday/Wednesday. It has an orbiter but it is to study earth and maybe does not have a camera at all maybe looking to the moon.nothing that I have seen could be described as a real photo showing anything worthwhile. It would not surprise me it both craft failed to soft land
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
It's all a farcical fake. The Chinese 'space station' videos are a hoot.Well one is certainly kaput and the latest is that the Indian attempt is Tuesday/Wednesday. It has an orbiter but it is to study earth and maybe does not have a camera at all maybe looking to the moon.
Why, could it have been sabotaged?I am not so sure about that. I think the craft crashed.
- Joined
- Sep 11, 2021
- Messages
- 9,803
- Reaction score
- 6,942
Surely it could have its electronics fried by a lazer beam.
But even without interference, a soft landing is nearly impossible imo, especially remotely.
Let's see it the dots have better luck in a few days.
But even without interference, a soft landing is nearly impossible imo, especially remotely.
Let's see it the dots have better luck in a few days.
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
It's difficult to find, but I once saw claimed Mars Rover video with a fly landing on the rover and walking on its surface. All filmed on Devon Island, Canada.
- Joined
- Jul 1, 2023
- Messages
- 5,039
- Reaction score
- 6,877
Same here, I heard the story but never saw anything to back it up.It's difficult to find, but I once saw claimed Mars Rover video with a fly landing on the rover and walking on its surface. All filmed on Devon Island, Canada.
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
YouTube changed their algorithms a while ago so you can't find older video unless you know the exact name. Accounts have years worth of video, with most no longer scrollable by date. They are still on YouTube , just not visible in the account catalogue.Same here, I heard the story but never saw anything to back it up.
Last edited:
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Who filmed the landing? Apparently this didn't appear an important question to the Chinese
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
Seriously, the Indian CGI team took the day off today, clearly. So bad!
- Joined
- May 2, 2022
- Messages
- 3,692
- Reaction score
- 4,956
Mr spoon went to button moon in the 80's in his converted beans tin and funnel

Agreed from what we can see so far, how was such camera control achieved, why no dust or shaking from such an obvious heavy landing??Who filmed the landing? Apparently this didn't appear an important question to the Chinese
Also I must agree with your criticism of the recorded footage presented from most of the missions, the images can hardly be taken seriously and are poorly explained from source.
Cheers!
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
The irony here is that Button Moon cosmology is likely closer to reality than the NASA fairy-tale
- Joined
- Jan 11, 2023
- Messages
- 4,205
- Reaction score
- 3,958
The vid has similar qualities to a 50's animation cartoon and again - Whose doing the camera work? and where is the touchdown?
It looks daft, so far.
Latest Threads
-
-
-
-
Is this whole Jeffrey Epstein thing a psyop ?
- Started by Jay Homer Simpson
- Replies: 16
-
The SSPX are going to consecrate new bishops
- Started by scolairebocht
- Replies: 5
Popular Threads
-
-
-
-
-
C
-
